I. Introduction
This protocol describes methods for covalently binding ligands to hydroxyl particles. Since hydroxyl groups are not spontaneously reactive with biomolecules, they must be activated using a variety of methods. Most activation strategies are carried out under nonaqueous conditions because the activating agent and the reactive intermediate group are typically susceptible to hydrolysis.
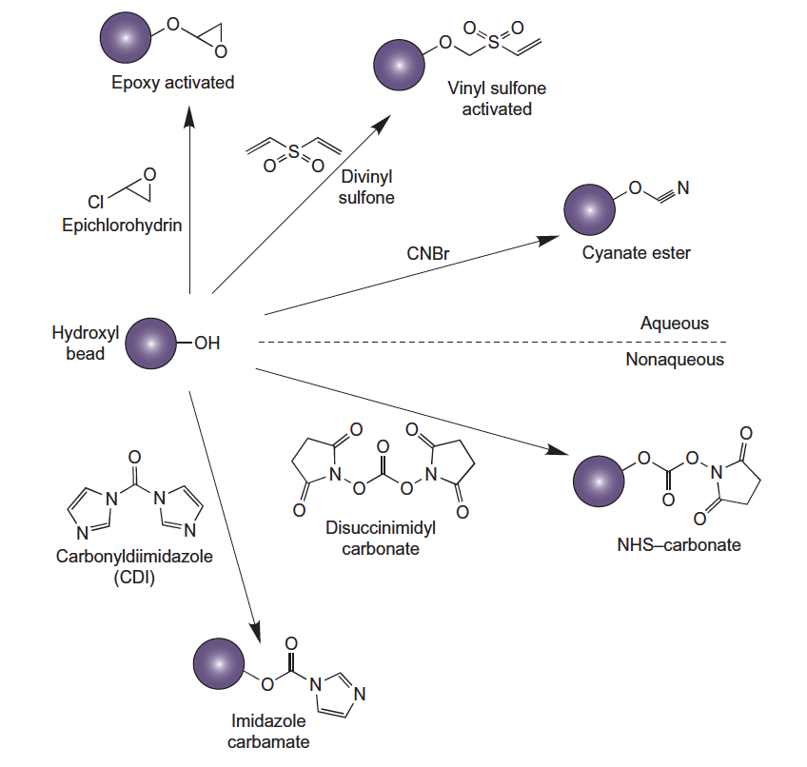
Figure.Hydroxyl-containing particles conjugation
II. Materials
- Hydroxyl-containing particles, such as those made from copolymers or composites of poly(hydroxyethyl methacrylate) (pHEMA).
- Anhydrous solvent, such as tetrahydrofuran (THF) or dimethylformamide (DMF).
- Activating agent: such as Carbonyldiimidazole (CDI) or Disuccinimidyl carbonate (DSC).
- Coupling buffer, appropriate for the ligand.
- Amine-containing ligand (e.g., protein or antibody).
- Quenching agent, such as ethanolamine or Tris buffer.
- Suitable storage buffer with a preservative.
III. Activation and Coupling Protocols
The following are some common methods to activate hydroxyl particles:
A. Activation Using CDI
- Solvent Exchange: Exchange pHEMA particles into anhydrous THF using centrifugation and resuspension with sequential exchange into greater percentages of THF in water until the particles are suspended in 100% THF. Wash several times with anhydrous THF to eliminate any remaining traces of water, letting the particles agitate in solvent between each centrifugation step. After the final THF wash centrifuge the particles and remove excess solvent.
- CDI Activation: Resuspend the particles in a solution of CDI in anhydrous THF. The concentration of CDI and the reaction time should be optimized for the specific particles and ligand.
- Washing: Wash the activated particles with anhydrous THF to remove excess CDI and reaction byproducts.
- Coupling: Resuspend the activated particles in an aqueous buffer containing the amine ligand to be coupled. The coupling reaction can be carried out at room temperature or 4°C with mixing for at least 2 hours or twice as long at 4°C.
- Quenching: Block any remaining active groups by adding ethanolamine or Tris buffer.
- Washing and Storage: Wash the particles with buffer to remove unreacted protein and quenching agent. Finally, suspend the particles in a suitable storage buffer containing a preservative.
B. Activation Using DSC
- Solvent: Ensure the hydroxyl-containing particles are in a solvent that will not deleteriously affect particle integrity.
- DSC Activation: Resuspend the particles as a 5% suspension in anhydrous solvent containing DSC at a concentration of 50 mg/ml (0.2-M).
- Reaction: React with mixing for 2 h at room temperature.
- Washing: Wash the activated particles three times with anhydrous solvent to remove excess DSC and reaction byproducts. After the final wash, remove the solvent and perform a quick wash with ice-cold water to remove most traces of solvent in the particle pellet.
- Coupling: Immediately resuspend the particles at a concentration of 10 mg/ml in 0.1-M sodium phosphate, pH 7.2 (coupling buffer), containing 1 to 10 mg of a protein or antibody and mix to dissolve. Alternatively, add the protein to the particle suspension in an amount equal to 1- to 10-times molar excess over the calculated monolayer for the protein type to be coupled. The optimal level of protein to be added should be determined experimentally.
- Reaction: React with mixing for at least 2 h at room temperature or twice as long at 4°C.
- Quenching: Add ethanolamine to the particle suspension at a final concentration of 0.1-M to quench any remaining active groups and react with mixing for 1 h. Other amine-containing quenchers may be used, too, such as Tris buffer. Note: DSC-activated sites on the particles that completely hydrolyze will revert back to the original hydroxyls.
- Washing and Storage: Centrifuge and wash the particles at least three times with buffer to remove unreacted protein and quenching agent. Finally, suspend the particles in a suitable storage buffer containing a preservative.
C. Cyanogen Bromide Activation
Caution: Cyanogen bromide is extremely toxic and should be used only in a well-ventilated fume hood while using appropriate personal protective gear.
- Washing: Wash 100 mg of hydroxyl-containing particles two times using centrifugation with 0.1-M sodium carbonate, pH 8.5 (coupling buffer). After the second wash, resuspend the particles at 10 mg/ml in 2-M sodium carbonate (activation buffer; no pH adjustment necessary).
- Protein preparation: Dissolve an amount of protein in 10 ml of coupling buffer equal to 1- to 10-times excess over the calculated monolayer for the type of particles being used.
- Activation: In a fume hood, dissolve 1 g of cyanogen bromide in 0.5 ml of acetonitrile (highly toxic!).
- Add a drop of the cyanogen bromide solution at a time to the particle suspension with constant mixing at room temperature. The entire solution should be added to the particles over the course of about 10 s.
- Activate the particles with mixing for exactly 2 min at room temperature.
- Quickly wash the particles with ice-cold deionized water and then with a volume of cold coupling buffer. Resuspend the particles in the protein solution prepared in step 2.
- Reaction: React for 24 h at 4°C with mixing.
- Wash the particles with coupling buffer and block excess reactive groups by resuspending in 50-mM ethanolamine, pH 9.0. React for 1 h at room temperature with mixing.
- Thoroughly wash the particles with storage buffer (e.g., PBS, pH 7.5, or other suitable buffer) and resuspend them at 10 mg/ml in storage buffer containing a preservative.
IV. Considerations and Optimization
- Solvent Compatibility: Ensure that the solvent used for activation does not adversely affect the particle integrity.
- Protein Concentration: Optimize the protein concentration to achieve the desired surface coverage without causing aggregation. A suitable starting set of trial experiments would employ protein concentrations in the range of about 10 to 200 μg protein per mg particles.
- Reaction Time and Temperature: Adjust the reaction time and temperature to optimize conjugation efficiency while preserving biomolecule activity.
- Quenching: Use appropriate quenching agents (e.g., ethanolamine) to block unreacted sites.
- Washing: Ensure thorough washing to remove unreacted reagents and prevent nonspecific binding.
V. Notes
- When using cyanogen bromide, all operations should be performed in a fume hood.
- The optimal conditions for each step may need to be determined experimentally for each specific ligand and particle type.
- The degree of protein loading can be determined using a protein assay technique such as the bicinchoninic acid (BCA) protein assay.
- For more information, consult the immobilization technology literature for affinity chromatography using larger hydroxyl-containing porous beads.
Interested in learning more about our particles with versatile functional groups for your conjugation? Visit Our Functional Polystyrene Particles to explore more.
Reference: Bioconjugate Techniques, 3rd Edition - July 25, 2013, Greg T. Hermanson
