I. Introduction
This protocol describes a method for coupling ligands containing thiol, amino, or hydroxyl groups to epoxide-containing particles via a ring-opening reaction that is facilitated under alkaline conditions. This reactive group can be used to couple proteins, nucleic acids, sugars and carbohydrates, and other organic molecules containing these functionalities.
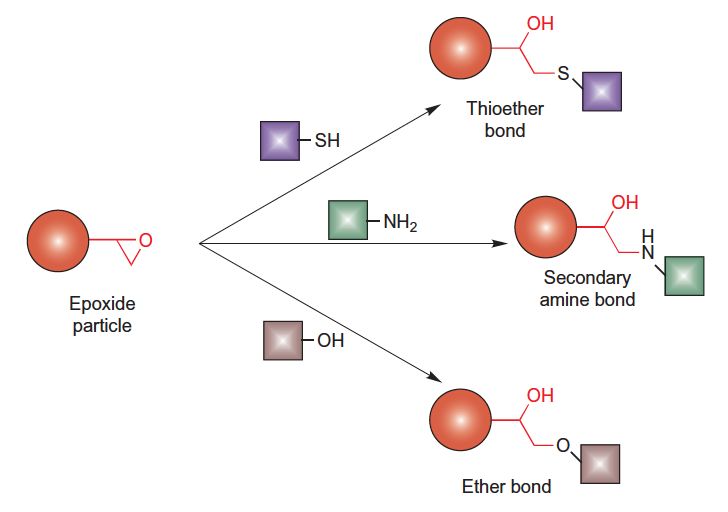
Figure.Particles containing reactive epoxy groups conjugation
II. Materials
- Epoxy particles
- Coupling buffer (i.e., 0.1-M sodium carbonate, pH 10, for coupling amine-containing ligands)
- Ligand to be coupled, containing thiol, amine, or hydroxyl groups
- Blocking agent, such as cysteine
- Storage buffer with a preservative
III. Protocol
- Wash epoxy particles with coupling buffer. Use higher pH conditions if coupling hydroxylic molecules and lower pH for coupling thiol-containing ligands. Suspend the particles at a 5% solution in coupling buffer.
- With mixing, add to the particle suspension a quantity of ligand dissolved in coupling buffer in an amount that represents a 1- to 10-times excess over the molar quantity of epoxide groups present on the particles.
- React at room temperature (for sensitive ligands) or at 45 to 60°C (for more stable ligands) for at least 20 h with mixing.
- Block excess epoxy groups by the addition of cysteine to a final concentration of 50 mM. Other small molecules can be used for blocking, provided they will efficiently react with the excess epoxides and not result in a modification that could interfere with the subsequent use of the particles. Continue the reaction with mixing for at least 2 h.
- Wash the particles thoroughly with coupling buffer and then into a more moderate pH storage buffer containing a preservative.
IV. Considerations and Optimization
- pH Adjustment: Adjust the pH of the carbonate coupling buffer according to the type of ligand being coupled. Use a slightly basic pH (pH 7.5–8.5) for thiol-containing ligands, higher pH values (pH 9–11) for amine-containing ligands, and very high alkaline conditions (pH > 11) for hydroxyl-containing ligands.
- Ligand Quantity: Use a quantity of ligand that represents a 1- to 10-times excess over the molar quantity of epoxide groups present on the particles.
- Temperature: React at room temperature for sensitive ligands or at 45 to 60°C for more stable ligands.
- Blocking: Use appropriate blocking agents to block excess epoxy groups.
- Washing: Ensure thorough washing to remove unreacted reagents and prevent nonspecific binding.
- Lyotropic Salts: The use of lyotropic salts may allow protein coupling to occur with excellent yield at pH 7.0 to 8.0, which is much more amenable to maintain protein stability.
- Protein Stability: Some proteins may require longer reaction times to reach maximal coupling yields for attachment to the support.
V. Notes
- The optimal conditions for each step may need to be determined experimentally for each specific ligand and particle type.
- The degree of protein loading can be determined using a protein assay technique.
- Epoxide groups can be introduced into polymeric particles through free-radical copolymerization with oxirane-containing vinyl monomers, such as allyl glycidyl ether or glycidyl methacrylate, or they may be introduced by surface modification using a bis-epoxide compound, such as 1,4-butanediol diglycidyl ether.
- Epoxy activation has been used extensively to immobilize affinity ligands onto porous beaded chromatography supports and can be used with equal success to couple ligands to microparticles and nanoparticles.
Interested in learning more about our particles with versatile functional groups for your conjugation? Visit Our Functional Polystyrene Particles to explore more.
Reference: Bioconjugate Techniques, 3rd Edition - July 25, 2013, Greg T. Hermanson
